Kinesis QTUG™
Kinesis QTUG™ - A proven tool for falls risk assessment. QTUG™ is intended to assist those assessing falls risk, by providing a falls risk score.

Quantitative methods for estimating falls risk or predicting falls have been suggested as a more objective means to identify patients at risk of falling before a fall, suitable both for use by experts and non-experts. These methods can be used in primary, secondary or residential care settings for assessing and targeting of individuals at high risk of falls. An objective method for assessing falls risk (using for example wearable sensors), suitable both for use by non-experts and for deployment in a community care setting would find clinical application for screening and targeting of individuals at high risk of falls. As current methods (e.g. Physical Profile Assessment, Tinetti scale, Berg balance scale) for assessing falls risk and mobility are often driven by a subjective observation of the patient’s gait and mobility there is potential for variability in administration and results.
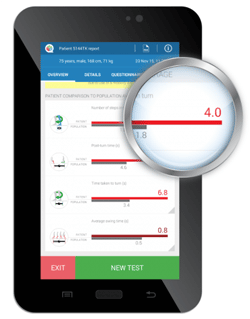
To address this variability, a new quantitative method for estimating falls risk (identifying patients at risk of falling) has been developed, known as the Quantitative Timed Up and Go (QTUG). QTUG™ is based on the Timed Up and Go test and is instrumented with wireless sensors placed on each leg. This technology provides a method for objective assessment of mobility and falls risk. It allows identification of a specific mobility impairment by comparison against a reference population in older adults.

Mobility and Falls Risk Assessment Technology.
QTUG™ is intended to assist those assessing falls risk, by providing a falls risk score (known as the Falls Risk Estimate (FRE)) along with fast, accurate and objective data. QTUG™ provides an automatic comparison of the patients gait and mobility against average values for their age and gender. QTUG™ also incorporates the American Geriatric Society (AGS) and British Geriatric Society (BGS) falls questionnaire which records standard falls risk-factors (such as polypharmacy, orthostatic hypotension, vision impairment etc). These data are used to improve the statistical falls risk estimate that QTUG™ provides. QTUG™ is a registered Class I medical device in the EU, under the Medical Device Directive and carries the CE mark. QTUG™ is registered with the FDA as a Class I (exempt) medical device in the USA, is registered as a Class I medical device with Health Canada, and is registered with the Therapeutic Goods Administration (TGA) in Australia. QTUG™ has been certified for electrical safety to medical device safety standard EN 60601-1.
NOTE: Content reproduced with permission from Kinesis Health Technologies Ltd.
